Volume 69, Issue 2 (April 2020)
Review Series: The start of a new era of biologics for treating allergic diseases
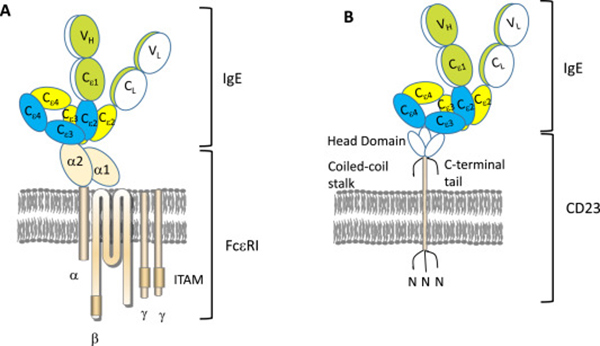
Profs. Okayama, Matsumoto, Odajima, Hide, and Okubo summarize the roles of IgE in allergic conditions, about 10 years of experience of omalizumab for severe allergic asthma, and treatment of chronic spontaneous urticaria and severe Japanese cedar pollinosis by omalizumab. Notably, omalizumab is useful in childhood as well as in adult asthma.
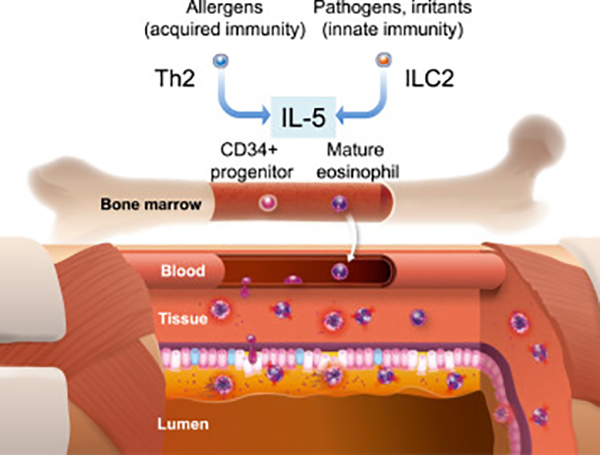
Profs. Nagase, Ueki, and Fujieda explain the roles of eosinophils in allergic diseases and application. They also describe the clinical trials of three IL-5/IL-5R antagonists mepolizumab, reslizumab, and benralizumab to asthma, EGPA, and chronic rhinosinusitis with nasal polyp. The success story of mepolizumab is a pioneering work of stratified medicine in allergic diseases. Moreover, it is interesting to know how different it is to target IL-5 versus IL-5R to deplete eosinophils.
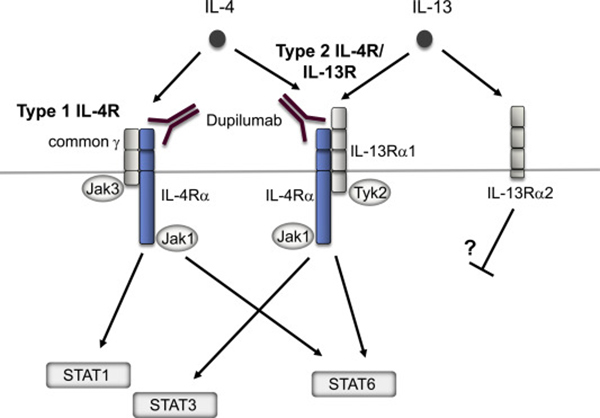
Profs. Matsunaga, Kato, Fujieda and Izuhara explain basic aspects of dupilumab regarding IL-4 and IL-13 signals and its application to allergic diseases. Dupilumab was the first biologic approved for atopic dermatitis and chronic rhinosinusitis with nasal polyp and is also approved for uncontrolled asthma. Many clinical trials are now underway for other allergic diseases in which the IL-4 and IL-13 signals are thought to play an important role.
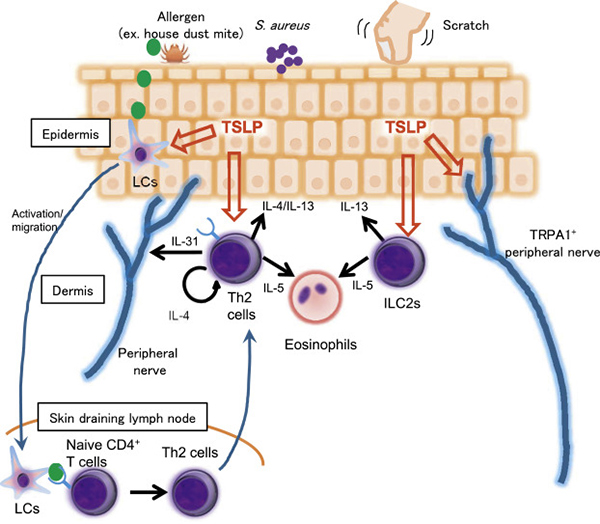
Profs. Kabashima and Asano report the role of TSLP as a master regulator of type 2 inflammation and application of tezepelumab, anti-TSLP Ab, for atopic dermatitis and asthma. The phase 2 study for moderate to severe atopic dermatitis was completed with promising but not fully satisfactory results, and the phase 3 study for severe asthma is in progress. It is of note that in the phase 2 study for asthma patients, tezepelumab showed efficacy irrespective of type 2 or non-type 2 biomarkers of the patients.